“Microbial alchemy is what we're
doing, transforming gold from something that has no value into a solid,
precious metal that's valuable”
Kazem Kashefi, Assistant Professor of Microbiology and
Molecular Genetics at Michigan State University Thursday October 4th
201
I
will always be surprised by the ability of bacteria to do amazing things at the
nanoscopic level. They can convert molecules from one form to the other, such
as sugars into alcohol, organic material into Methane and even hydrogen as
mentioned sparing in my blog
article entitled “UTECH
partners with GOJ and UWI to develope Hydrogen Cooking Gas Cylinders - EU
Funded 3 Year Project is Chasing Mavericks to push Jamaica into the
Hydrogen-Electron Economy”.
But
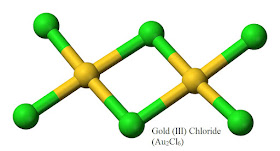
I’d never imagine a class of bacteria, Cupriavidus metallidurans existed that could convert toxic
Gold (III) Chloride (Au2Cl6) into pure Gold (Au(s))
nuggets after seven (7) days as stated in the article “Bling! Researchers create 24k gold
in the lab”,
published October 4, 2012 4:13 PM PDT by Christopher MacManus, CNET
News. This is Biochemistry at its best
folks, as I’m for the first time speechless and in awe of the potential of this
discovery, despite its impracticality as a commercial process.
Professor Kazem Kashefi and
Associate Professor Adam Brown are the evil geniuses behind this development.
Their work was on display at the Prix Ars Electronica cyber Art competition in
Austria between Thursday October 4th 2012AD to Sunday October 7th 2012AD.
Aptly titled “The Great Work of the
Metal Lover”, it consisted of 24-Karat Gold-themed equiptment that was being
used in their research, a kind of Counter culture Art-Meets-Science exhibition
consisting of a Glass bioreactor and the Cupriavidus
metallidurans bacteria as shown below.
This is pure Biochemistry Art tinged
with strong commercial possibility, to quote Associate Professor Adam Brown:
“This is neo-alchemy. Every part, every detail of the project is a cross
between modern microbiology and alchemy. Science tries to explain the
phenomenological world. As an artist, I'm trying to create a phenomenon. Art
has the ability to push scientific inquiry”.
This process may even go a long way
to explain why panning for Gold (Au(s)) was even possible, as
presumably this bacterium was present in rivers and is responsible for Gold (Au(s))
nuggets in river beds. Gold (Au(s)), despite its relative
unreactiveness as a Transition Metal, normally occurs in nature as a chemically
combined Ore.
The regular process for extracting Gold
(Au(s)) involves dissolving it in Sodium Cyanide (HCN) that is used
to produce an inorganic complex that precipitates out of solution and is easily
separated by using Distilled Water:
4 Au(s) + 8
NaCN(aq) + O2(g) + 2 H2O(l)
→ 4 Na[Au(CN)2](s) + 4 NaOH(aq)
According to the articles “Gold(III) chloride”, visited November 10, 2012, Wikipedia
and “Aqua regia”,
visited November 10, 2012, Wikipedia, Gold
(III) Chloride (Au2Cl6) used as the food for the Cupriavidus metallidurans is produced via one of two (2) methods in the
Laboratory.
The first is via oxidation of Gold
(Au(s)) using Chlorine Gas (Cl2) at 180 C at s.t.p
(Standard Temperature and Pressure):
2
Au(s) + 3 Cl2(g) → 2 AuCl3(s)
The second method involves several
steps and is actually commercially viable with Ore with Gold (Au(s)).
Gold (Au(s)) ore is reacted with Aqua Regia, a 1:3 ratio of
concentrated Nitric Acid (HNO3) and Hydrochloric Acid (HCl) to
produce Gold (Au(s)) with a valence of 3+ i.e. Au3+(aq).
Aqua Regia is a mixture usually used
to produce Gold (Au(s)) of the highest quality:
Au(s) + 3 NO−3(aq)
+ 6 H+(aq) →Au3+(aq) + 3 NO2(g)
+ 3 H2O(l)
Then the Au3+(aq) is
reacted with a Chloride Salt in the presence of Nitric Acid (HNO3)
to produce Gold Tetrachloride (AuCl3(aq)) :
Au3+(aq) + 3
NOCl(g) + 3 NO−3(aq) → AuCl3(aq) +
6 NO2(g)
AuCl3(aq) + Cl-(aq)
→AuCl−4(aq)
Add a little heat, and Gold (III)
Chloride (Au2Cl6) is the final product:
2 HAuCl4(s) → Au2Cl6(s)
+ 2 HCl(g)
The final stage involves using the above process instead
involves no Hydrogen Cyanide (HCN) and
directly converts Gold
(III) Chloride (Au2Cl6) directly into Gold (Au(s)):
Au2Cl6(l) + Cupriavidus
metallidurans =
Au(s)
By mere cursory glance, the
practicality of making Gold (Au(s)) via this method is revealed
using Cupriavidus metallidurans
bacteria! The reagents are a bit expensive, to be sure; protection from and
recovery of Chlorine Gas (Cl2(g)) will be very critical to the
safety of staff at such a Plant and its overall success. But Economy-of-scale
and the current prevailing high price of Gold (Au(s)) on the
Mercantile Exchange Markets should make it worth the while for an adventurous
Investor to pursue the extraction of this noblest of Metals via this purely
Organic, albeit corrosive method.
I’d love to see a scaled up version
of this in Jamaica used to mine Gold, a venture in which the GOJ has shown
interest in resuming back in July 2011AD in Pennants, Clarendon as noted in my
blog
article entitled “AUSJAM and the Pennants Gold Mine
Rush - King Solomons Mines in Clarendon”. A revival of Gold Mining in Clarendon surely to bring out
the prospectors in a Mining for Gold in Clarendon, Jamaica by a Bio-Chemistry Step Up Revolution (2012) for Cyanide-Free Gold Mining!

Great! You mentioned that the "reagents"were expensive. Do you have any info concering possible costs?
ReplyDelete