“Increasing
environmental and supply security issues are leading the aviation sector to
seek alternative fuels which can be used interchangeably with today's jet fuel,
so-called drop-in solution. With this first-ever proof-of-concept for 'solar'
kerosene, the SOLAR-JET project has made a major step towards truly sustainable
fuels with virtually unlimited feedstocks in the future”
Project Coordinator at
Bauhaus Luftfahrt, Dr. Andreas Sizmann commenting on the potential of the
SOLAR-JET Project's use of the Fischer–Tropsch Process to make Kerosene from Carbon
Dioxide, Water Vapour and Sunlight
 |
All
thanks to a Research Team from EU (European Union)-backed SOLAR-JET Project
finally figuring out a practical way to implement the well-known Fischer–Tropsch
Process, which was invented by the chemists Franz Fischer and Hans Tropsch
in Germany in the mid 1920s.
The
SOLAR-JET (Solar chemical reactor demonstration and Optimization for Long-term
Availability of Renewable JET fuel) was kickstarted in June 2011 with financial
support for the next four (4) years from the EU (European Union) within the 7th
Framework Programme. Their success in producing Syngas is now a
Proof-of-concept idea that will put the European Union in the forefront of
research and production of Fossil Fuels directly from concentrated solar energy,
effectively making it a renewable Resource.
The
SOLAR-JET Project as described in “Synthesized
'solar' jet fuel: Renewable kerosene from sunlight, water and Carbon Dioxide”,
published May 3 2014 by ETH Zurich, Science Daily consists of the following
players, who all have a common goal of producing a sustainable way of making
Fossil Fuels using Sunlight:
1.
Bauhaus Luftfahrt
2.
Deutsches Zentrum für Luft- und
Raumfahrt (DLR)
3.
ARTTIC
4.
Shell Global Solutions
5.
Swiss University ETH Zurich
This
by overcoming two technical problems that have dogged the practical
implementation of the Fischer–Tropsch
Process in the production of Synthetic Gas of Syngas for short:
1.
Disassociation of Carbon Dioxide (CO2(g))
and Water (H2O(g)) into Hydrogen (H2(g)), Carbon
Monoxide (CO(g)) and Oxygen (O2(g)) using Sunlight
2.
Removal of Oxygen (O2(g))
from the reaction, as its presence causes the Fischer–Tropsch Process reactor
vessel to Explode
Production
of SynGas was always the problem for the well established Fischer–Tropsch
Process to produce Kerosene. This was the hurdle that the SOLAR-JET Project
had overcome. Granted, the Research Team from the SOLAR-JET Project only made
one glassful of Kerosene. Still, we humans now have perfected the Fischer–Tropsch
Process, a tool that can close the Krebb Cycle and Carbon Dioxide (CO2(g))
Cycle sans Plants and algae or
genetically modified Bacteria as described in my blog article
entitled “Pyrococcusfuriosus
Bacterium Bio-engineered by University of Georgia’s to convert Carbon Dioxide
to Bio-fuel - Carbon Sequestering profitable
Hunger Games Catching”!
But
depending on how you view your glass, it can be half full of potential or half
empty promises of a future where any Fuel can literally, be made out of thin
Greenhouse Gases in the Air.
Fischer–Tropsch Process
– Syngas and how Oxygen is only good if it’s Oprah Winfrey’s Channel
So
how did they do it? First watch the video below as it explains the production
of Syngas feedstock to be used in the Fischer–Tropsch
Process to produce Kerosene.
The
uses concentrated Solar Radiation to convert Carbon Dioxide (CO2(g))
and Water Vapour (H2O(g)) into Syngas. Syngas is a
mixture made up purely of Hydrogen (H2(g)) and Carbon Monoxide (CO(g))
and can be produced from the disassociation of Carbon Dioxide (CO2(g))
and Water Vapour (H2O(g)) as described in “‘Solar’
jet fuel made out of thin air”, published 2 May 2014 by Jon Cartwright, Chemistry World.
There
is a problem however.
Carbon
Dioxide (CO2(g)) and Water Vapour (H2O(g))
only disassociate at temperatures of about 2200°C in the first process. The
SOLAR-JET Team overcame this problem by using a high-flux solar simulator at the
Swiss university ETH Zurich, a fairly simple thing to do. Thus Carbon Dioxide
(CO2(g)) and Water Vapour (H2O(g)) becomes Hydrogen
(H2(g)), Carbon Monoxide (CO(g)) and Oxygen (O2(g))
as per the equation below.
CO2(g)
+ H2O(g) →H2(g) + CO(g) + O2(g)
For
the next stage, this mixture of Hydrogen (H2(g)), Carbon Monoxide
(CO(g)) and Oxygen (O2(g)) now contains Carbon Monoxide
(CO(g)), Hydrogen (H2(g)) and Oxygen (O2(g)).
To remove the Oxygen (O2(g)) and thus convert it to Syngas, which is
made up purely of Hydrogen (H2(g)) and Carbon Monoxide (CO(g)),
SOLAR-JET Team employed the use of Cerium(IV) Oxide (CeO2) commercially
known as Ceria (CeO2) to
remove the Oxygen (O2(g)).
 |
Based
on my knowledge of making Bamboo Charcoal using Vacuum Pyrolysis as described
in my blog
article entitled “Jamaica's
Bamboo Charcoal exports stalled by lack of Bamboo Furnaces – How to build a
Fresnel Lens Solar Powered Bamboo Furnace and produce Activated Charcoal
byproduct”, they could have used a Fresnel Lens to focus Sunlight unto the
Quartz Glass to achieve temperatures of 2200°C
When
Ceria (CeO2) is heated to about 1500°C in a vacuum by concentrated
sunlight, Ceria (CeO2) is reduced, releasing Oxygen (O2(g)).
Once the Oxygen (O2(g)) is removed from the Second Stage Process
Chamber, the Ceria (CeO2) is ready to remove Oxygen (O2(g))
from the disassociated Carbon Monoxide (CO(g)), Hydrogen (H2(g))
and Oxygen (O2(g)) mixture from the first part of the process.
This
Second Stage Process is closely monitored for the presence of Oxygen (O2(g))
so as to avoid a massive explosion due to the reaction of the Hydrogen (H2(g))
and the Oxygen (O2(g)), which can result as the disassociated Carbon
Monoxide (CO(g)), Hydrogen (H2(g)) and Oxygen (O2(g))
mixture is very hot from the Radiation Energy from the Solar Concentrator and
highly reactive.
 |
Ceria
(CeO2) can again be reduced using concentrated sunlight back to its Oxygen-Free
form, ready for reaction. Because the Disassociation of Carbon Dioxide (CO2(g))
and Water Vapour (H2O(g)) are produced in the same
connected chamber and then rapidly cooled from 2200°C to 1500°C to allow the
Ceria (CeO2) to absorb Oxygen (O2) from the reaction.
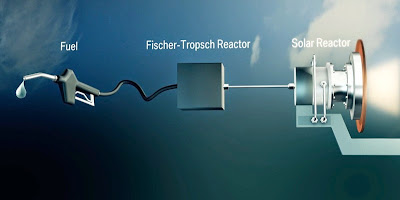
Once
fully reacted, the resulting Syngas passes out the bottom of the outlet chamber
and is piped unto the Third Stage Process, the Fischer–Tropsch
Process, from which Kerosene is the end result. You can see the video below
that explains the Fischer–Tropsch
Process.
20,000
Liters of Jet Fuel a day is sure to make many Developed Countries with large
Military covet this technology. For Developing World Countries, it could mean
the end for the need to import Fossil Fuel and may result in them being able to
manufacture their own fuel. It’s a fairly easy petrochemical Engineering
Process to convert Kerosene, a lighter faction extracted from the Catalytic
Cracking of Petroleum to heavier fuel oils such as Gasoline and Diesel.
Syngas on Tap for
Fischer–Tropsch Process – Fossil Fuel a Renewable Resource by 2017
So
with this process, it’s been demonstrated that you can literally make Kerosene
or Jet Fuel from the same material that plants use in photosynthesis to make Sugars.
The feedstock is unlimited too, as our current consumption of Fossil Fuels
means that there will be plenty of Carbon Dioxide (CO2(g)) and Water
(H2O(g)) to fuel Syngas production to quote one of the
researchers on the SOLAR-JET Project at the German think-tank Bauhaus
Luftfahrt, Dr. Andreas Sizmann: “Sunlight, Carbon Dioxide and water are
basically an unlimited feedstock. When the long term goal of 15% overall energy
efficiency is reached, 20,000 litres of kerosene per day could be produced in a
solar tower system of one square kilometre.”
Best
of all, without having to convert Bio-Mass via the use of Bacteria such as the genetically
modified bacterium, Pyrococcusfuriosus as described in my blog article
entitled “Pyrococcusfuriosus
Bacterium Bio-engineered by University of Georgia’s to convert Carbon Dioxide
to Bio-fuel - Carbon Sequestering profitable
Hunger Games Catching”.
Granted
they only made a cup full of Kerosene, with the efficiency of Sunlight to
Syngas Production being rated at 1.73%. Still this is a very significant
milestone and the stage is set to achieve economy-of-scale according to Dr.
Andreas Sizmann, quote: “This is an extremely important milestone in the
long-term process of developing a truly sustainable alternative fuel future.
The process [draws] from virtually unlimited resources with no prohibitive cost
“show stopper” in sight”. Ramping up the efficiency of the Syngas Process is a
matter of improving the following:
1.
Improvements in materials used
2.
Reactor geometry
3.
Heat management
4.
Gas management
5.
Reactor Size
According
to Máire Geoghegan-Quinn, European commissioner for research, innovation and
science, the Oil Industry will probably get turned upside down as this can
potentially provide fuel for all Vehicles on Earth, quote: “This technology
means we might one day produce cleaner and plentiful fuel for planes, cars and
other forms of transport. This could greatly increase energy security and turn
one of the main greenhouse gases responsible for global warming into a useful
resource”.
Shell,
a major Oil Drilling company with Petrochemical services geared at producing
various products from Oil, is also involved in the SOLAR-JET Project, hopefully
with an interest to expand research, not stifle it to quote Professor Hans
Geerlings at Shell: “This is potentially a very interesting novel pathway to
liquid hydrocarbon fuels using focused solar power. Although the individual
steps of the process have previously been demonstrated at various scales, no
attempt had been made previously to integrate the end-to-end system. We look
forward to working with the project partners to drive forward research and
development in the next phase of the project on such an ambitious emerging
technology”.
Let’s
hope that as other Scientists replicate SOLAR-JET Process of making Syngas
around the world, we’ll get a lot closer to this dream of Fossil Fuels as a
Renewable Resource by 2017.
No comments:
Post a Comment